When we talk about aldehydes in the context of perfumery what is usually meant is the group of odorous chemicals more accurately called saturated aliphatic aldehydes. There are in fact quite a lot of other aldehydes routinely used in perfumery including such commonplace materials as vanillin (the key component in vanilla) but in this post I'm focussing on that particular group with the characteristic aroma, described by perfume people as 'aldehydic'.
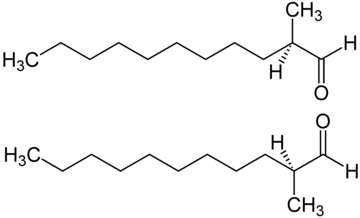 |
| The structure of C12 MNA - one of the key aldehydes used in perfumery |
Besides being olfactorily interesting these materials also have some special characteristics from the perspective of storage and use. All are very powerful and hence tend to be used in very low doses but they can have a very high impact on the scent. Several are found in nature, notably in citrus oils where they contribute to the bright, sparkling, fresh nature of those materials.
Here are some details I put together in response to a question about what exactly they are and how they should be stored and handled:
What are Aliphatic Aldehydes?- Octyl aldehyde (AKA Aldehyde C8 or octanal)
- Nonyl aldehyde (AKA Aldehyde C9 or nonanal)
- Decyl aldehyde (AKA Aldehyde C10 or decanal)
- Undecenyl aldehyde (AKA Aldehyde C11 undecylenic;Aldehyde C11 enic; hendecenal or Aldehyde C111)
- Undecylic aldehyde (AKA Aldehyde C11 undecylic or undecanal)
- Lauric aldehyde (AKA Aldehyde C12 lauric or dodenanal)
- Methyl nonyl acetaldehyde (AKA Aldehyde C12 MNA or 2-methyl undecanal)
Aliphatic aldehydes (saturated) are the ones most people mean when they talk about aldehydes in the context of perfume:
But notice that the so-called Aldehyde C14 (AKA Peach Aldehyde or gamma-Undecalactone), Aldehyde C16 (Strawberry glycidate or lots of alternate names) and Aldehyde C18 (Coconut Aldehyde or gamma-Nonalactone) are not on the list because they are not in fact aldehydes at all*.
There are lots of other aldehydes but they don't exhibit the same special characteristics for storage purposes as the saturated aliphatic aldehydes - the ones listed above are the main ones used in perfumery, though there are a few others used by some manufacturers as captives*.
All the saturated aliphatic aldehydes are best diluted in a primary alcohol* as soon as you get them - in alcohols they form hemi-acetals which smell like the aldehyde they are formed from but are much more stable (in solution). Personally I like to keep my aldehydes at 1% for blending purposes but for storage 10% is more practical as it keeps the volume you have to store more manageable.
If you keep them neat they tend, over time, to either oxidise into the corresponding acids, which smell nasty; or polymerise into trimers, which have no smell at all. The presence of any acid (including oxidation products) will accelerate the trimer production significantly. The really counterintuitive point is that trimers continue to form at very low temperatures and seem to form faster, so you should keep your aliphatic aldehydes at room temperature until you've diluted them in alcohol.
You'll know if your aldehydes have trimerised because, besides smelling less strong than they should, they will have become thicker & will eventually be solid at room temperature as the trimer has a much higher melting point.
Adding BHT or other anti-oxidant can help with both problems, though won't eliminate them completely. There is also some evidence that they keep better in aluminium than in glass.
At Pell Wall we’ve found in practice that by far the worst culprit for trimer formation is Aldehyde C12 Lauric. It’s so bad that we don’t sell the undiluted material at all: as soon as fresh stock arrives we dilute it to 10% in ethanol and benzyl alcohol and sell it in that form so that it remains in good condition.
* a captive is a molecule that is either patented by or kept secret by a manufacturer for use only in their own fragrances.
*the obvious primary alcohol to use is ethanol (or Perfumer’s Alcohol), since you are probably going to use them in an alcoholic fragrance in the end anyway, but phenyl ethyl alcohol or benzyl alcohol work too and might be preferable in some applications.
Much of the information in this post is sourced from Perfume and Flavouring Synthetics by Paul Z Bedoukian
There are lots of other aldehydes but they don't exhibit the same special characteristics for storage purposes as the saturated aliphatic aldehydes - the ones listed above are the main ones used in perfumery, though there are a few others used by some manufacturers as captives*.
All the saturated aliphatic aldehydes are best diluted in a primary alcohol* as soon as you get them - in alcohols they form hemi-acetals which smell like the aldehyde they are formed from but are much more stable (in solution). Personally I like to keep my aldehydes at 1% for blending purposes but for storage 10% is more practical as it keeps the volume you have to store more manageable.
If you keep them neat they tend, over time, to either oxidise into the corresponding acids, which smell nasty; or polymerise into trimers, which have no smell at all. The presence of any acid (including oxidation products) will accelerate the trimer production significantly. The really counterintuitive point is that trimers continue to form at very low temperatures and seem to form faster, so you should keep your aliphatic aldehydes at room temperature until you've diluted them in alcohol.
You'll know if your aldehydes have trimerised because, besides smelling less strong than they should, they will have become thicker & will eventually be solid at room temperature as the trimer has a much higher melting point.
Adding BHT or other anti-oxidant can help with both problems, though won't eliminate them completely. There is also some evidence that they keep better in aluminium than in glass.
At Pell Wall we’ve found in practice that by far the worst culprit for trimer formation is Aldehyde C12 Lauric. It’s so bad that we don’t sell the undiluted material at all: as soon as fresh stock arrives we dilute it to 10% in ethanol and benzyl alcohol and sell it in that form so that it remains in good condition.
Footnotes
* these materials came to be called Aldehydes because when they were first discovered the manufacturer wanted to conceal their chemical identity and so deliberately mis-named them to confuse those who might want to copy the new molecules. It helped that they had a comparable power to the true aliphatic aldehydes. In many quarters the names have stuck and they are still widely referenced and sold as aldehyde C14, aldehyde C16 and aldehyde C18.* a captive is a molecule that is either patented by or kept secret by a manufacturer for use only in their own fragrances.
*the obvious primary alcohol to use is ethanol (or Perfumer’s Alcohol), since you are probably going to use them in an alcoholic fragrance in the end anyway, but phenyl ethyl alcohol or benzyl alcohol work too and might be preferable in some applications.
Much of the information in this post is sourced from Perfume and Flavouring Synthetics by Paul Z Bedoukian

Thanks for posting this experience. Which temperature do you suggest for storage and to use it? Plastic (PEHD) is it good to storage?
ReplyDeleteMy Aldehyde C12 Lauric turned completely solid. Is there anything I can do?
ReplyDeleteThere’s nothing you can do with Lauric Aldehyde once it’s gone solid because it means it has polymerised. It’s because this happens so easily that we only sell this particular aldehyde at 10% in either ethanol or benzyl alcohol, which keeps it stable. https://pellwall.com/shop/ingredients-for-perfumery/liquids/aldehyde-c12-lauric-10/
DeleteIts cold here in Colorado, I just received my sample of Lauric aldehyde from PSH and it was a white solid that turned back to liquid upon warming. I have since diluted in 200 ethanol. Does that mean it is bad? Or when you say it is bad when solid, does that mean a permanent state of solid?
DeleteI suggest room temperature and I would not use plastic - aluminium or glass are much better
ReplyDeleteCan we mix these Aldehydes with essential oils and absolutes in making perfumes, without any adverse reactions?
ReplyDeleteThanks for the article. It was v informative.
It can do damge to your health if you smell them from the bottle?
ReplyDelete